We offer a full array of key services in our
Services
Each day we work to advance innovative manufacturing
technologies that promise cures, making cell and gene
therapies more accessible to everyone.
Process Development
We offer an end-to-end solution for viral vector manufacturing, from cell line to drug product, emphasizing process robustness and quality. Our platform approach minimizes new development, allowing for the rapid creation of toxicology batches shortly after contract signing. With Quality by Design (QbD) principles, we transition directly to GMP production, saving you time and resources for study design.
cGMP Manufacturing
Our commitment to quick clinic delivery and accurate execution is fundamental to our approach. With an integrated supply chain and standardized documentation strategies, we significantly reduce GMP readiness timelines and maintain a sharp focus on the CMC specifics of your program.
Analytical Development
Our analytical services are focused on viral vector product release, emphasizing biological assays crucial for quality approval. Our team is equipped to handle everything from assay transfer to qualification and validation, aiming to effectively bring your ideas to life.
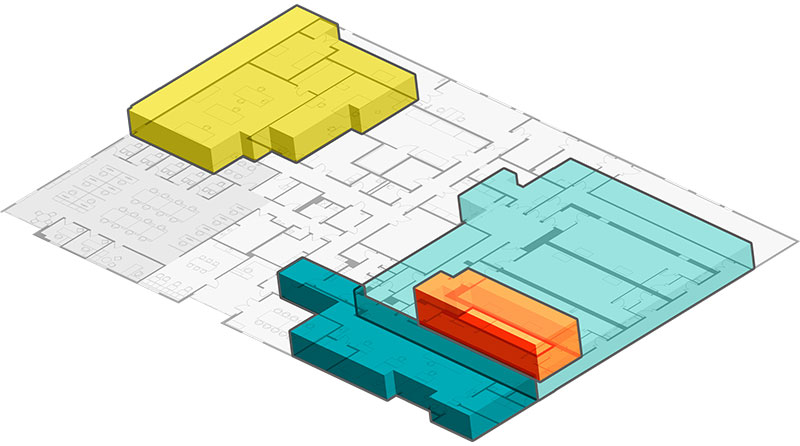
Explore the facility
Facility details
22,434 sq ft
BSL2- ISO7/ISO8
5 dedicated, cGMP suites
Dedicated Process Development
& Toxicology (GLP)
Dedicated RUO Vector Core
On-site Warehouse
& Cold-Chain Storage
24/7 environmental monitoring of
production suites and cold storage
Integrated Analytical Suite
Quality is at the core of what we do
Our comprehensive quality assurance program means that
viral vector products are manufactured and tested in full
compliance with cGMP regulations and adhere to FDA Good
Manufacturing Practice (GMP) requirements.
With more than 40 years of quality assurance program
management, our team has adopted a “right first time”
culture, dedicated to proactive quality program
management to ensure the quality of your product.